As9100 Internal Audit Checklist Template
Implementing any standard can become very stressful at a company. Sometimes it seems everyone has his or her own idea of how it should be done. When we do not provide the proper level of communication to the employees, they become frustrated with the company.
When upgrading or implementing a new system it is important to let the employees know the plans for the company. By upgrading to AS9100 it will open doors that otherwise would remain closed. The aerospace market is booming with potential new customers and clients. TECH TIPS As long as you have management support you will not have problems with the implementation of your quality system. It is very important that you communicate to the employees what you are doing and why you are doing it.
If executive management and the employees buy into the system, it will reduce the amount of resistance that you receive from them. Employees become angry and they do not understand why they have to become certified to a standard.
Layered Process Audit Templates – Bing images Audit Checklist Template needed. Quality Audit Checklist Template. Audit forms lean manufacturing audit manufacturing audit checklist AS9100 Internal Audit Checklist 7.5 Production and Service. Financial Audit Checklist – To Do List, Organizer, Checklist, PIM.

If we communicate to them, it will help but it will not be 100%. You will still have employees who do not like change or do not want to change.
We have all heard it; the employees say the following statements. • We have always done it this way, why do we need to change? • Our customers like our product the way we are making it, why do we have to change our system? • We are making our delivery to our customer on time. Why do we have to change? • Why does everything have to be approved before we use it? • Why do changes have to be approved before using the documents?
Some companies like to dive into the standard and blindly write an overabundance of procedures, work instructions, or training manuals. They try to interpret as they go through the standard. Other companies will hire a consultant to put a canned system in place. They will charge you heavily for this canned system. The canned system will not be uniquely written for your company. Lastly, others will put a team together to determine what is needed and to break up the requirements and distribute the workload.
You need to have your executive management on board for a successful implementation of any new quality system. If your upper management is not onboard, you will have a very difficult time getting them to buy into all of the changes. If you do not already have an organization chart, you need to create one. You should get your executives and department heads together to discuss the standard and the implementation process. If you do not have their buy in you will not be successful in your implementation process.
My views and takeaways from AS9100C and other quality systems, including ISO 9001, TS 16949, ISO 13485, etc., are: If it says you shall.that means you must do what it asks for. If the standard says, you shall have a procedure for a clause you have to have a documented procedure.
A documented procedure means that the document has a unique tracking number and a revision date, revision level or both. The procedure must be approved before being released for use. I suggest you create a log. The log will be used to track your documents and their revision level. Excel is a great tracking tool that I have used successfully. Keep it simple: document number, title, revision level, revision date, the location and who owns the documents. Create tabs at the bottom of the worksheet for your procedures, forms, and work instructions.
AS9100C Requirements The focus for this article will be on the Aerospace AS9100 Revision C. For my example, the company is already certified to ISO 9001:2008.
The AS9100C standard calls out for a minimum of six procedures. Just because a procedure is not required does not mean that your company does not need to have documented instruction on how you perform an operation. Procedures ensure that each person is doing the task the same way each and every time. These six procedures must be documented and controlled by your quality system. They will be a part of your Excel log discussed earlier. A documented procedure is required for the following areas: 1.
Control of Documents; 2. Control of Records; 3. Internal Audits; 4. Control of Nonconforming Product; 5. Corrective Actions; 6. Preventative action.
Procedures can be a flow chart of the process or they can be just a word document. Read the standard and make sure that everything that is required is covered in the procedure. Remember your procedure has to have a unique number, revision level, and who approved the procedure for use. The flow chart method is my preferred method. It is very precise and to the point without a lot of reading. Here are samples of a word-documented procedure and a flow chart documented procedure. Sample using the Word document method.
Sample of a required procedure using the flow chart method is on the next page. The buttons on the sides of the document are hyperlinks that take you to the other documents that are referenced. AS9100C IMPLEMENTATION This article will teach you how to implement AS9100C in a stress free environment. After implementation, you will meet the requirements of the standard while still having a simple compliant system. Remember KISS: Keep It Simple, Silly. Personally, I have implemented over fifty quality systems.
I have developed five easy steps and used them throughout decades of successfully implementing quality systems. Preparation 3. Documenting 5.
Corrective Actions PLANNING The first step is PLANNING. At the planning stage, you will ask a series of questions. Often times this stage is performed by just one or two key people within the organization. You need to answer the following questions truthfully. As you are answering the questions clearly document your answers. • What do we want to do?
• How do we want to do it? • When does it have to be done?
• Who will be on the team? PLANNING example: What do we want to do? We want to implement AS9100C How do we want to do it? We want to do it quickly and efficiently. We want to do it without a lot of stress. We do not want to avoid disruptions to the production facility.
When does it have to be done? Our customer is specifying that we be in compliance in ten months.
Then become certified by a third party registrar within twelve months. Who will be on the team?
We need a cross functional team. A representative from quality, purchasing, manufacturing, and human resources is an excellent team. Who will lead the team? For this article, the quality manager will lead the team PREPARATION The second step is PREPARATION. During this step you will review the answers to your questions from step one.
Purchase the AS9100 Rev C checklist and the AS9100C standard. Confer with your cross functional team and either split up the checklist or have one person complete the entire checklist during step four. Personally, I like to audit the entire system myself and complete the checklist. I take the checklist and type it into Excel and create a template like the one shown.
I add in when areas will be started and when they will be completed. I have a proposed section and an actual date section.
TEACHING The third step is TEACHING the employees about the standard that you will be implementing. Give them an overview of the AS9100C standard.
We are not making them auditors so an overview is all that is needed. Touch on the basics and let them know the team will be out asking questions in order to complete the checklist. If you look at the standard, everything that is in bold is new to AS9100C. Most likely, we will have to create documents to become compliant.
I take the standard with the employees in a small group of ten and go through an overview of the key points of what will be added to the existing system. I have found that communication is very important. Remember most of the population does not like change and if we can even get a few to buy into the system, it will help morale during the implementation stages. If you do not communicate to them, they may get the wrong idea of what you are doing. None of us likes someone looking over our shoulder and writing thing down. Free Download Mp3 Nike Ardila Full Album Rar.
Communicate during the teaching session that you will be taking many notes and asking many questions. DOCUMENTING The forth step is DOCUMENTING. At this step, you have your checklist typed into a spreadsheet, printed and on a clipboard. You are ready to audit the entire system for compliance or to discover noncompliance. At this stage it is better that we find the noncompliance and fix them before the external auditor audits the company.
When you find things that do not meet the standard write them down on the checklist. When you find that we are in compliance write that down too. I like to audit and complete my checklist by section. As you are going through the completion of the checklist, you will find that it asks the same type of question in more than one area.
Start filling out the checklist and making notes that support the compliance status. S is used for satisfactory, NC is for noncompliance. For example as we are looking at the checklist, we see that the first item is: A QMS (Quality Management System) has been established, documented, implemented, and maintained with evidence of continual improvement. Under Compliance, it has an S meaning it is compliant. Objective evidence to support this is a quality manual dated 11/1/2013. The next example is Item # 3, the requirement is that the QMS addresses customer, statutory and regulatory requirements, under compliance, we see that this is an NC meaning that we are noncompliant.
The objective evidence to support this is that it is not addressed in the quality manual. We continue through all of the pages of the checklist until it is completed, gathering our information to prove compliance or noncompliance. AS9100C has eight sections. The first three sections are Scope; Normative References; and Terms and Conditions.
There are five main sections and under each section, there are clauses. The main sections start at four and run through eight. Quality Management System 5. Management Responsibility 6. Resource Management 7. Product Realization 8. Measurement, Analysis and Improvement CORRECTIVE ACTION The fifth step is CORRECTIVE ACTION.
Corrective Actions are issued after you document and complete your checklist. Every place you have an NC you have to create a corrective action plan. Evaluate and determine areas that you are lacking in your system. Start an Excel spreadsheet to document your results and to issue Corrective Action Requests (CARs). Take the Excel implementation plan and sort by the NCs.
Now take the NC and copy and paste them into a corrective action spreadsheet. I like to use the 8d method for corrective actions. This method goes through all of the steps to create a solid corrective action. After completing the checklist and completing the corrective actions that are needed we can now begin our implementation phase. Make sure that the six procedures that are required have been documented and approved. After all of the Corrective Actions are completed and all of the clauses are addressed you now have a compliant AS9100C quality system.
Start using the system right away so that you can begin to have records to prove compliance. This audit that was just completed is called your Implementation Internal Audit, keep all of the documentation including the CARs together because this will count as your first Internal Audit to AS9100C. You should have three to six months of documented use before your external third party audit. About three months before the audit, repeat the audit and document your results onto the checklist. After you complete the checklist, you should have very few NCs.
Do not be discouraged if you find something that is not compliant. Put corrective action into place to correct the issue and bring it into compliance. Also, if you discover that you implemented something and it is not being done go through it with whoever is responsible and have them sign off on a training record that they understand. Maybe the process that you set up is not working properly. The quality system is a living set of documents. We continually improve upon the quality system to make it better.
You can create and give tests to prove compliance to the CAR. In conclusion, this is my view on implementing a stress free quality system.
It does not matter which standard you are implementing. The process is the same for all of them. Five steps to stress-free implementation are preparation, planning, teaching, documenting, issuing and implementing corrective actions. As long as you have management support you will not have problems with the implementation of your quality system. It is very important that you communicate to the employees what you are doing and why you are doing it. If executive management and the employees buy into the system, it will reduce the amount of resistance that you receive from them. They will provide the support that is necessary for a successful implementation.
Always keep it simple, to the point and in compliance of the standard you are implementing. Good luck on your next quality system implementation.
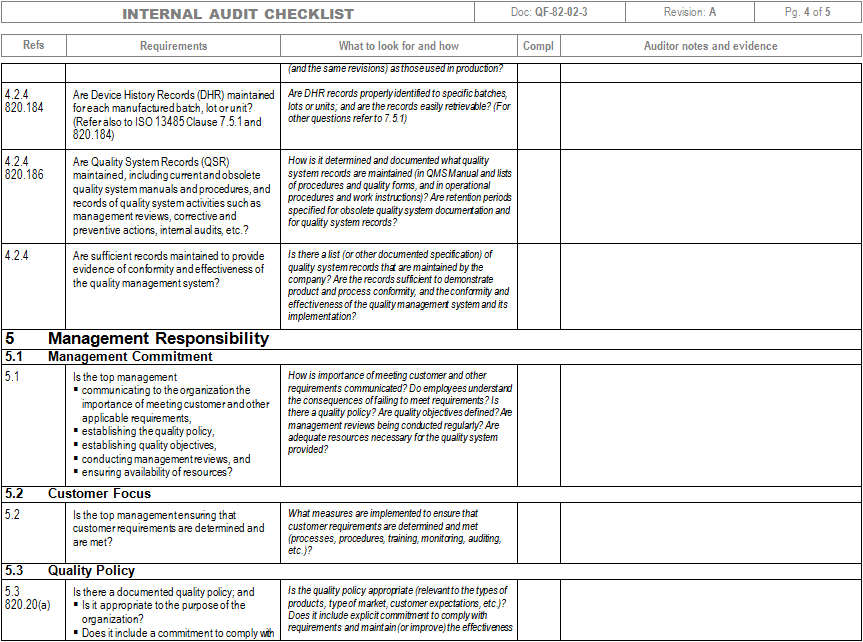
Internal Quality Audits Internal Quality Audits: OP-822-01 Revision D Effective Date:4/30/2015 Owner Approval by Brian L. Darby, AS9100 Coordinator, on 4/30/2015 Management Approval by Craig O. Garneau, President, on 4/30/2015 Printed copies of this document are for information purposes only and are uncontrolled. Printed copies are not valid after the date of printing.
This document was printed on • PURPOSE To provide a consistent procedure for conducting internal quality audits. • RESPONSIBILITY The table below identifies the people and their responsibility relating to this procedure: Person Responsibility President Quality Assurance Manager AS9100 Coordinator Establishes the schedule for performing internal quality audits. Designates trained internal auditors to perform and document quality system audits.
Reviews and evaluates internal audit results at appropriate intervals. Department Supervisors Quality Assurance Manager AS9100 Coordinator Responds to internal audit findings with appropriate corrective action. Trained Internal Auditors Perform internal audits. • APPLICABLE FORMS OR REFERENCES The table below identifies the number and the title of the forms and references that are applicable to this procedure; Form/Reference Number Title AS9100 Quality Management Systems - Requirements for Aviation, Space and Defense Organizations N /A Internal Audit Check List N/A • POLICIES. • Audits will be conducted using a process approach. All processes determined in our quality manual will be covered per the audit schedule.
Baixar Musica Da Banda Strike Ceu Completo there. The purpose of the audits is to determine whether QNP's QMS conforms to our own planned arrangements and requirements, the requirements of the AS9100 standards, and our customers' contractual requirements. • The audit schedule - shall be established so that compliance with all processes is audited once a year, at a minimum. The audit schedule and frequency shall be adjusted in response to quality system priorities that are identified by QNP management and based on the status and importance of the activity. The Audit Cycle Schedule and Audit Plan records are maintained in the online and printed on FM-822-01 Audit Cycle Schedule/Audit Plan. • Audits will be documented on an audit finding report either electronically or by hard copy. • Debriefing - The area/department supervisor will be debriefed on the audit results after the audit is completed. This shall be communicated by the assigned auditors as a verbal summary.
Findings will be reviewed by the AS9100 Coordinator or Quality Assurance Manager, after which a copy of the completed Audit Finding Report will be available upon request. • Audit findings (non-compliances) recorded on the Audit Finding Report must be detailed and specific enough to provide the responsible area/department supervisor with complete assessment information.
These findings shall be documented as defined below. • Audit findings – These shall consist of: • C= Conformances - Evidence was found that process meets requirement.
• OBS= Observations - Slight or minor deviation from process does not have a major impact on process or would not allow nonconforming product to escape. • NC= Major Non-conformance – This is written if a number of OBS are found of similar nature or if a systemic breakdown is occurring. NC’s are written when a significant failure is found to the specified requirement.
Also can be written if the failure or the breakdown can cause nonconforming product to escape. • Review - Audit Finding Reports are reviewed by the AS9100 Coordinator or the Quality Assurance Manager. They will review the findings for accuracy. They shall discuss the audit results with the auditor(s) and determine if the non-conformances written require corrective action requests (CAR’s).
• Issuing Corrective Action Request (CAR)- If an audit results in findings, and the AS9100 Coordinator or Quality Assurance Manager determine it necessary, use to initiate corrective action. If an audit finding does not warrant a full corrective action, follow up activities may be documented within the, that is maintained within the. • Management Review - Internal audit status and results shall be reported during all Management Review Meetings. The AS9100 Coordinator or Quality manager shall report the following: • completion status of the internal audits • a summary of recent audit results • the status of audit corrective actions • changes to Quality Policy Manual, procedures, or work instructions • actions required • Issuing Audit Assignments - The President, AS9100 Coordinator and/or the Quality Manager is responsible for reviewing the audit plan /schedule for the coming months and issuing audit assignments to trained internal auditors.
Internal Auditors can be subcontracted consultants in addition to employees. • Trained Auditors- Auditors are required to have evidence of completing a two (2) day internal or external training class given by an approved/certified auditor to RABQSA or IRCA in the past three years. An Internal Auditor could also be c onsidered trained if they hold an active certification from the above. If a fully trained auditor is not available, management may designate the responsibility to personnel with an appropriate amount of experience and knowledge of the auditing process.
• All recorded audit results- shall be listed on an Audit Finding Report even if there are no findings /non-compliances. This audit record will provide evidence that the audit was performed. • Internal Audit Checklists may vary, but should be similar to the current Process Effectiveness Assessment Reports used by Registars or per customer requirements (i.e ASQR-01 Form 1) • Outputs include- evidence of completed audits, corrective actions if required, retention of records. Audit results will be an input to Management Review Meetings. • Timely Corrective Action: QNP management responsible for the area audited shall ensure that corrections and corrective actions are taken without undue delay to eliminate the found nonconformities and their causes.
The amount of time appropriate to initiate and complete a corrective action request shall depend of the nature and severity of the non-conformance, the scope of the action required, and the risk that QNP's customers may receive non-conforming product. Conducting Internal Quality Audits Step Action Person(s) Responsible 1. Prepare the audit schedule, and communicate schedule to auditors. President, Quality Assurance Manager, and/or AS9100 Coordinator 2. Prepare audit checklist.
Give audited departments and employees advance notice of audit to be conducted. Internal Auditor AS9100 Coordinator 3. Conduct audit using process approach. Internal Auditor 4.
Communicate audit results to the audited employees and management. Internal Auditor 5.
Issue Corrective Action Requests as necessary based on audit results. President, Quality Assurance Manager, and/or AS9100 Coordinator 6. Review audit results during periodic management review meetings. President, Quality Assurance Manager, and/or AS9100 Coordinator • RECORDS/OBJECTIVE EVIDENCE The retention duration for records referenced in this procedure are available on-line in the Records are maintained in accordance with. • REVISION HISTORY Revision, approved date, effective date, and a document change summary for this document is located on-line in the.